Πώς τον πετσόκοψες έτσι;
Τι έλεγαν οι φορουμίτες στην αρχή της πανδημίας
Κανόνες Δ. Συζήτησης
Οι πληροφορίες, οι συμβουλές και γενικότερα το υλικό που δημοσιεύεται σε αυτή την ενότητα έχουν αποκλειστικά ενημερωτικό χαρακτήρα και εκφράζουν τις προσωπικές απόψεις των αρχικών συγγραφέων στα πλαίσια της δημόσιας συζήτησης. Σε καμία περίπτωση δεν αποτελούν επιστημονική ιατρική πληροφόρηση. Το Phorum.com.gr δεν παρέχει ιατρικές συμβουλές, ούτε φέρει ευθύνη για το υλικό που δημοσιεύεται εδώ ή σε άλλη ενότητα της κοινότητας, ή μεταφέρεται ως πληροφορία με τη χρήση προσωπικών μηνυμάτων, e-mail και άλλων τρόπων. Δεν φέρουμε καμία απολύτως ευθύνη για οποιαδήποτε τυχόν σωματική / ψυχική βλάβη λόγω εσφαλμένης πληροφόρησης. Συστήνουμε πάντοτε να συμβουλεύεστε γιατρό για θέματα υγείας.
Η ανάγνωση ή/και η συμμετοχή σας στην παρούσα ενότητα συνεπάγεται ότι συμφωνείτε και αποδέχεστε ανεπιφύλακτα τους παραπάνω όρους.
Οι πληροφορίες, οι συμβουλές και γενικότερα το υλικό που δημοσιεύεται σε αυτή την ενότητα έχουν αποκλειστικά ενημερωτικό χαρακτήρα και εκφράζουν τις προσωπικές απόψεις των αρχικών συγγραφέων στα πλαίσια της δημόσιας συζήτησης. Σε καμία περίπτωση δεν αποτελούν επιστημονική ιατρική πληροφόρηση. Το Phorum.com.gr δεν παρέχει ιατρικές συμβουλές, ούτε φέρει ευθύνη για το υλικό που δημοσιεύεται εδώ ή σε άλλη ενότητα της κοινότητας, ή μεταφέρεται ως πληροφορία με τη χρήση προσωπικών μηνυμάτων, e-mail και άλλων τρόπων. Δεν φέρουμε καμία απολύτως ευθύνη για οποιαδήποτε τυχόν σωματική / ψυχική βλάβη λόγω εσφαλμένης πληροφόρησης. Συστήνουμε πάντοτε να συμβουλεύεστε γιατρό για θέματα υγείας.
Η ανάγνωση ή/και η συμμετοχή σας στην παρούσα ενότητα συνεπάγεται ότι συμφωνείτε και αποδέχεστε ανεπιφύλακτα τους παραπάνω όρους.
Re: Τι έλεγαν οι φορουμίτες στην αρχή της πανδημίας
Occasio facit furem
Εγώ δεν είμαι χριστιανός, για να μην πω πως είμαι και άθεος!
Ο κόσμον ασοί γραμματισμέντς θα διαβαίν.
Εγώ δεν είμαι χριστιανός, για να μην πω πως είμαι και άθεος!
Ο κόσμον ασοί γραμματισμέντς θα διαβαίν.
Re: Τι έλεγαν οι φορουμίτες στην αρχή της πανδημίας
γιαννη γιαννοπουλε βγες απο τον λογαριασμο του χελλεγγενης
γιαννοπουλονημα
γιαννοπουλονημα
- hellegennes
- Δημοσιεύσεις: 45155
- Εγγραφή: 01 Απρ 2018, 00:17
Re: Τι έλεγαν οι φορουμίτες στην αρχή της πανδημίας
Εσύ πάντως είσαι σταθερός. Από την αρχή έλεγες ότι ακόμα και 1 θάνατος είναι καταστροφή. Μπορεί να μην έχει επαφή με την πραγματική πραγματικότητα, που δεν είμαστε όλοι Χαϊλάντερ, αλλά τουλάχιστον δεν αναπροσαρμόζεις την καταστροφολογία σου ανάλογα με το πόσο έχεις πέσει έξω.
Ξημέρωσε.
Α, τι ωραία που είναι!
Ήρθε η ώρα να κοιμηθώ.
Κι αν είμαι τυχερός,
θα με ξυπνήσουν μια Δευτέρα παρουσία κατά την θρησκεία.
Μα δεν ξέρω αν και τότε να σηκωθώ θελήσω.
Α, τι ωραία που είναι!
Ήρθε η ώρα να κοιμηθώ.
Κι αν είμαι τυχερός,
θα με ξυπνήσουν μια Δευτέρα παρουσία κατά την θρησκεία.
Μα δεν ξέρω αν και τότε να σηκωθώ θελήσω.
Re: Τι έλεγαν οι φορουμίτες στην αρχή της πανδημίας
Κοιτάξτε δεν ξέρω αν φαντάζεστε ότι τα τεστ τα κάνει η κυβέρνηση. Μερικά δειγματοληπτικά όπως στις πλατείες ναι. Αλλά γενικά τεστ κάνει μόνο όποιος θέλει να κάνει και έχει να πληρώσει ή όποιος έχει πάρε-δώσε με νοσοκομεία, που είναι και η μόνη περίπτωση στην οποία καλύπτονται (αν πρέπει π.χ. να κάνεις μια επέμβαση). Το τεστ δεν είναι υποχρεωτικό ούτε καν για αδερφάκι κρούσματος προκειμένου να πάει σχολείο. Το έμαθα σήμερα με το χειρότερο τρόπο.
Και βέβαια η κυβέρνηση δεν ασχολείται ούτε να το κάνει υποχρεωτικό, ούτε να το προσφέρει, ούτε να ελεγξει την τιμή του ούτε τίποτα. Είναι ολοφάνερο ότι δε βιάζονται να εμφανίσουν πολλά κρούσματα.
Τώρα 20.000 ακούγεται εντυπωσιακό όμως πρώτον όπως είπα η συντριπτική πλειοψηφία είναι άτομα που έκαναν με δικιά τους πρωτοβουλία και έξοδα και δεύτερον αν το ανάγεις στην αγαπημένη σου μετρική (ανά εκατομμύριο πληθυσμού) πρόκειται για μια αθλιότητα.
Re: Τι έλεγαν οι φορουμίτες στην αρχή της πανδημίας
Αυτό δεν το ξέρεις για τα τεστ; https://www.ethnos.gr/ygeia/127595_koro ... covid-poiafoscilis έγραψε: ↑15 Οκτ 2020, 21:46
Κοιτάξτε δεν ξέρω αν φαντάζεστε ότι τα τεστ τα κάνει η κυβέρνηση. Μερικά δειγματοληπτικά όπως στις πλατείες ναι. Αλλά γενικά τεστ κάνει μόνο όποιος θέλει να κάνει και έχει να πληρώσει ή όποιος έχει πάρε-δώσε με νοσοκομεία, που είναι και η μόνη περίπτωση στην οποία καλύπτονται (αν πρέπει π.χ. να κάνεις μια επέμβαση). Το τεστ δεν είναι υποχρεωτικό ούτε καν για αδερφάκι κρούσματος προκειμένου να πάει σχολείο. Το έμαθα σήμερα με το χειρότερο τρόπο.
Και βέβαια η κυβέρνηση δεν ασχολείται ούτε να το κάνει υποχρεωτικό, ούτε να το προσφέρει, ούτε να ελεγξει την τιμή του ούτε τίποτα. Είναι ολοφάνερο ότι δε βιάζονται να εμφανίσουν πολλά κρούσματα.
Τώρα 20.000 ακούγεται εντυπωσιακό όμως πρώτον όπως είπα η συντριπτική πλειοψηφία είναι άτομα που έκαναν με δικιά τους πρωτοβουλία και έξοδα και δεύτερον αν το ανάγεις στην αγαπημένη σου μετρική (ανά εκατομμύριο πληθυσμού) πρόκειται για μια αθλιότητα.
Σε κάθε περίπτωση πάντως, εγώ ποτέ δεν είπα ότι στην Ελλάδα σκίζουμε στα τεστ αν και πλέον γίνονται πολύ περισσότερα.
Αυτό που λέω είναι ότι βάσει των επίσημων στοιχείων ο απολογισμός ως προς τα θύματα είναι καλός, συγκριτικά με ότι γίνεται στην Ευρώπη, στα Βαλκάνια, στη Μεσόγειο. Αν εσένα δεν σου αρέσει αυτή η μετρική, δεν μπορώ να κάνω κάτι για αυτό.
- omg kai 3 lol
- Δημοσιεύσεις: 7453
- Εγγραφή: 03 Απρ 2018, 14:03
Re: Τι έλεγαν οι φορουμίτες στην αρχή της πανδημίας
foscilis έγραψε: ↑15 Οκτ 2020, 21:46Κοιτάξτε δεν ξέρω αν φαντάζεστε ότι τα τεστ τα κάνει η κυβέρνηση. Μερικά δειγματοληπτικά όπως στις πλατείες ναι. Αλλά γενικά τεστ κάνει μόνο όποιος θέλει να κάνει και έχει να πληρώσει ή όποιος έχει πάρε-δώσε με νοσοκομεία, που είναι και η μόνη περίπτωση στην οποία καλύπτονται (αν πρέπει π.χ. να κάνεις μια επέμβαση). Το τεστ δεν είναι υποχρεωτικό ούτε καν για αδερφάκι κρούσματος προκειμένου να πάει σχολείο. Το έμαθα σήμερα με το χειρότερο τρόπο.
Και βέβαια η κυβέρνηση δεν ασχολείται ούτε να το κάνει υποχρεωτικό, ούτε να το προσφέρει, ούτε να ελεγξει την τιμή του ούτε τίποτα. Είναι ολοφάνερο ότι δε βιάζονται να εμφανίσουν πολλά κρούσματα.
Τώρα 20.000 ακούγεται εντυπωσιακό όμως πρώτον όπως είπα η συντριπτική πλειοψηφία είναι άτομα που έκαναν με δικιά τους πρωτοβουλία και έξοδα και δεύτερον αν το ανάγεις στην αγαπημένη σου μετρική (ανά εκατομμύριο πληθυσμού) πρόκειται για μια αθλιότητα.
γιαυτο απο τα μεσα καλοκαιριου κι επειτα που αυξηθηκαν τα τεστ και τα κρουσματα ο πιο εγκυρος τροπος αναγνωσης ειναι το ποσοστο θετικων κρουσματων επι των ημερησιων τεστ
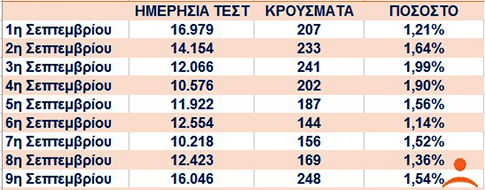
https://www.iatronet.gr/eidiseis-nea/ep ... nakas.html
Μία απλή μαθηματική πράξη δείχνει, παράλληλα, ένα ακόμα στοιχείο που δημιουργεί προβληματισμό. Το ποσοστό των θετικών κρoυσμάτων (217) επί των ημερησίων τεστ (3.808) την Κυριακή, 16 Αυγούστου, είναι 5,7% και σύμφωνα με υπολογισμούς είναι το υψηλότερο ποσοστό που έχει σημειωθεί από την αρχή της πανδημίας στην Ελλάδα.
Το αμέσως προηγούμενο μεγαλύτερο ποσοστό καταγράφηκε στις 13 Αυγούστου και ήταν το 2,7% θετικών επί συνόλου τεστ. Συγκεκριμένα, είχαν γίνει 7.553 τεστ και τα 204 ήταν θετικά. Τρίτο μεγαλύτερο ποσοστό (2,37%) ήταν την ημέρα που σημειώθηκε ρεκόρ κρουσμάτων στη χώρα μας, τα 262, όταν είχαν διενεργηθεί 11.046 τεστ το ίδιο 24ωρο. Αναλυτικά πόσα τεστ έγιναν:
12 Αυγούστου 2020: Εγιναν 11.046 τεστ, 262 εντοπίστηκαν θετικά (2,37% θετικών κρουσμάτων επί του συνόλου των ημερήσιων τεστ)
13 Αυγούστου 2020: Εγιναν 7.553 τεστ, εντοπίστηκαν 204 θετικά (2,70% θετικών κρουσμάτων επί του συνόλου των ημερήσιων τεστ)
16 Αυγούστου 2020: Εγιναν 3.808 τεστ, 217 εντοπίστηκαν θετικά (5,70% θετικών κρουσμάτων επί του συνόλου των ημερήσιων τεστ)
https://www.pagenews.gr/2020/08/17/ella ... ena-24oro/
Re: Τι έλεγαν οι φορουμίτες στην αρχή της πανδημίας
Αν το ποσοστό θετικών σε μία χώρα είναι υψηλός, αυτό δείχνει είτε ότι δεν γίνονται αρκετά τεστ και αυτά που γίνονται είναι μόνο επί των νοσούντων (παράδειγμα το Μεξικό που από την αρχή τής επιδημίας μέχρι σήμερα έχει ποσοστό θετικών 39,1%) ή ότι η εξάπλωση τής επιδημίας σε αυτή την χώρα είναι πολύ μεγάλη. Ομως και σε αυτή την περίπτωση θα έπρεπε να γίνονται περισσότερα τεστ, γιά να ιχνηλατηθεί η ασθένεια. Αρα, συμφωνώ ότι το ποσοστό των θετικών είναι από τα βασικότερα στοιχεία παρακολούθησης. Σε αυτό λοιπόν το ποσοστό, η Ελλάδα τον Οκτώβριο έχει ποσοστό θετικών 3,0% έναντι 2,1% π.χ. τής Γερμανίας. Αρα, θα πρέπει να δεχθούμε ότι κατ'αναλογία στην Ελλάδα γίνονται λιγότερα τεστ απ'ό,τι στην Γερμανία. Πράγματι, ανά εκατομμύριο πληθυσμού η Γερμανία έκανε μέχρι στιγμής 31.745 τεστ και η Ελλάδα 17.454 (δεν μετράω τα rapid tests αν και δεν ξέρω αν στην Γερμανία τα συμπεριλαμβάνουν και αυτά). Αυτό το νούμερο είναι το 55% εκείνου τής Γερμανίας, και πάντως όχι τόσο μικρό ώστε να χαρακτηρισθεί αθλιότητα, όπως λέει ο foscilis. Ομως: στις ΗΠΑ, το ποσοστό των θετικών είναι 5,0%. Οι ΗΠΑ έχουν υψηλότερο κατά κεφαλήν αριθμό τεστ, 42.317. Μεταξύ των δύο, ποιός θα λέγαμε ότι κάνει τα περισσότερα τεστ; Εγώ λέω η Γερμανία. Και πάμε παρακάτω: Η Ιταλία έχει ποσοστό θετικών 3,8%, το ΗΒ 5,2%, η Ισπανία 9,1%, το Βέλγιο 9,8% και η Γαλλία 11,5%. Τελικά, ποιός κάνει πολλά και ποιός λίγα τεστ;omg kai 3 lol έγραψε: ↑15 Οκτ 2020, 22:15γιαυτο απο τα μεσα καλοκαιριου κι επειτα που αυξηθηκαν τα τεστ και τα κρουσματα ο πιο εγκυρος τροπος αναγνωσης ειναι το ποσοστο θετικων κρουσματων επι των ημερησιων τεστfoscilis έγραψε: ↑15 Οκτ 2020, 21:46Κοιτάξτε δεν ξέρω αν φαντάζεστε ότι τα τεστ τα κάνει η κυβέρνηση. Μερικά δειγματοληπτικά όπως στις πλατείες ναι. Αλλά γενικά τεστ κάνει μόνο όποιος θέλει να κάνει και έχει να πληρώσει ή όποιος έχει πάρε-δώσε με νοσοκομεία, που είναι και η μόνη περίπτωση στην οποία καλύπτονται (αν πρέπει π.χ. να κάνεις μια επέμβαση). Το τεστ δεν είναι υποχρεωτικό ούτε καν για αδερφάκι κρούσματος προκειμένου να πάει σχολείο. Το έμαθα σήμερα με το χειρότερο τρόπο.
Και βέβαια η κυβέρνηση δεν ασχολείται ούτε να το κάνει υποχρεωτικό, ούτε να το προσφέρει, ούτε να ελεγξει την τιμή του ούτε τίποτα. Είναι ολοφάνερο ότι δε βιάζονται να εμφανίσουν πολλά κρούσματα.
Τώρα 20.000 ακούγεται εντυπωσιακό όμως πρώτον όπως είπα η συντριπτική πλειοψηφία είναι άτομα που έκαναν με δικιά τους πρωτοβουλία και έξοδα και δεύτερον αν το ανάγεις στην αγαπημένη σου μετρική (ανά εκατομμύριο πληθυσμού) πρόκειται για μια αθλιότητα.
https://www.iatronet.gr/eidiseis-nea/ep ... nakas.html
Ολα αυτά δεν έπρεπε να τα γράψεις, γιατί είναι παρωχημένα. Δυστυχώς, έχουμε χειροτερέψει σε σχέση με τον Αύγουστο που είχαμε ποσοστό θετικών 1,4% σε 2,3% τον Σεπτέμβριο και 3,0% τον Οκτώβριο. Πολύ περισσότερες μέρες με υψηλά ποσοστά θετικών υπήρξαν έκτοτε.omg kai 3 lol έγραψε: ↑15 Οκτ 2020, 22:15Μία απλή μαθηματική πράξη δείχνει, παράλληλα, ένα ακόμα στοιχείο που δημιουργεί προβληματισμό. Το ποσοστό των θετικών κρoυσμάτων (217) επί των ημερησίων τεστ (3.808) την Κυριακή, 16 Αυγούστου, είναι 5,7% και σύμφωνα με υπολογισμούς είναι το υψηλότερο ποσοστό που έχει σημειωθεί από την αρχή της πανδημίας στην Ελλάδα. Το αμέσως προηγούμενο μεγαλύτερο ποσοστό καταγράφηκε στις 13 Αυγούστου και ήταν το 2,7% θετικών επί συνόλου τεστ. Συγκεκριμένα, είχαν γίνει 7.553 τεστ και τα 204 ήταν θετικά. Τρίτο μεγαλύτερο ποσοστό (2,37%) ήταν την ημέρα που σημειώθηκε ρεκόρ κρουσμάτων στη χώρα μας, τα 262, όταν είχαν διενεργηθεί 11.046 τεστ το ίδιο 24ωρο. Αναλυτικά πόσα τεστ έγιναν:
12 Αυγούστου 2020: Εγιναν 11.046 τεστ, 262 εντοπίστηκαν θετικά (2,37% θετικών κρουσμάτων επί του συνόλου των ημερήσιων τεστ)
13 Αυγούστου 2020: Εγιναν 7.553 τεστ, εντοπίστηκαν 204 θετικά (2,70% θετικών κρουσμάτων επί του συνόλου των ημερήσιων τεστ)
16 Αυγούστου 2020: Εγιναν 3.808 τεστ, 217 εντοπίστηκαν θετικά (5,70% θετικών κρουσμάτων επί του συνόλου των ημερήσιων τεστ)
https://www.pagenews.gr/2020/08/17/ella ... ena-24oro/
1.
2. Η λέξη υδρόφιλος είναι σύνθετη από δύο ελληνικές λέξεις το ύδωρ που σήμαινε νερό και το φίλος που σήμαινε φίλος.
Re: Τι έλεγαν οι φορουμίτες στην αρχή της πανδημίας
Με δυο λογια ειμαι ο μονος που εχει σταθερα δικιο.
- Κεντροδεξιός Ψάλτης
- Δημοσιεύσεις: 1790
- Εγγραφή: 08 Σεπ 2019, 19:55
Re: Τι έλεγαν οι φορουμίτες στην αρχή της πανδημίας
Το ποσοστό θετικών δεν έχει καμμιά σημασία όταν γίνεται στοχευμένα μετά από ιχνηλάτηση.Αυτό που βρήκε κάποιος μια μέρα του Αυγούστου με ποσοστό 5.7% είναι ανοησίες.Προφανώς πρόκειται για μια μέρα που έκαναν τεστ μόνο σε αυτούς που έρχονταν στα νοσοκομεία με συμπτώματα.Το ποσοστό έχει νόημα μόνο όταν γίνεται με τυχαίο δειγματοληπτικό έλεγχο.Από τα rapid που γίνεται με αυτόν τον τρόπο το ποσοστό είναι κοντά στο 2%.
- Κεντροδεξιός Ψάλτης
- Δημοσιεύσεις: 1790
- Εγγραφή: 08 Σεπ 2019, 19:55
Re: Τι έλεγαν οι φορουμίτες στην αρχή της πανδημίας
Και πιο συγκεκριμένα δίκιο έχω.16 Αυγούστου πέφτει Κυριακή που δεν λειτουργούν τα ιδιωτικά και γίνονται τεστ μόνο από τα νοσοκομεία.Γι αυτό και Κυριακές με Δευτέρες το ποσοστό είναι ψηλό.
- unholydiver
- Δημοσιεύσεις: 389
- Εγγραφή: 01 Σεπ 2020, 21:31
Re: Τι έλεγαν οι φορουμίτες στην αρχή της πανδημίας
Απάντώντας σε άλλο φόρουμ σε κάποιον που στη συμπεριφορά μοιάζει με τον MEGA GNOSTI AGGLIKON του phorum
Δεν είπα ότι είναι γεμάτη ηλίθιους. Είναι πολλοί όμως και είναι πολύ χαμηλό το μορφωτικό επίπεδο τους. Μην επεκταθώ στα υπόλοιπα.
Δεν είπα ότι συμβαίνει μόνο στην Ελλάδα. Συμβαίνει παντού, αλλού περισσότερο αλλού λιγότερο.
Προφανώς δεν είπα ότι είμαι "απλοϊκός εξυπνάκιας". Δικός σου προσδιορισμός είναι, γιατί δεν σου άρεσε το κυνικό και όχι ελιτίστικο σχόλιο μου.
Είπα το απλό. Βλέποντας τι υπάρχει γύρω σου, ακόμα και σε forum, καταλαβαίνεις γιατί έχεις πετύχει, όπου έχεις πετύχει.
Το αν περνάω τον πήχη των ψεκασμένων 2 φορές ή 100 φορές δεν το ξέρεις. Σίγουρα όμως τον περνάω εύκολα.
Δεν λέω γιατρουδάκο τον Τσιόδρα και έχω την αντίληψη να ξεχωρίσω δημοσίευση στο "New England Journal of Medicine" από ψεκασμένα blog.
Όχι φίλε, σε όσους έχουν διαφορετική άποψη μας αποκαλείτε ηλίθιους και μας προσάπτετε διάφορα επίθετα όπως 5Gάκηδες, ανεγκέφαλους, αντιεμβολιαστές, αρνητές ιών ( έχετε μεγάλη φαντασία) φτωχαδάκια (έυγε) και ένα σωρό.
Αφού έχεις την αντίληψη να ξεχωρίσεις δημοσίευση στα περιβόητα ιατρικά journals σε σχέση με εμάς τους ψεκασμένους, πάρε κάτι ωραίες δηλώσεις από κάποιους ψεκασμένους συντάκτες των journals. Να με συγχωρήσετε όσοι δεν γνωρίζετε καλά αγγλικά γιατί δεν προλαβαίνω να μεταφράσω παρά μόνο την πρώτη δήλωση, είμαι κουρασμένος και αγχωμένος που θα ανοίξω αύριο το μαγαζί.
.Even the editors of main journals themselves recognise that peer review may not be the best system ever devised by mankind. Here is what Richard Horton, the editor of The Lancet, has to say on the matter:
“The mistake, of course, is to have thought that peer review was any more than a crude means of discovering the acceptability — not the validity — of a new finding. Editors and scientists alike insist on the pivotal importance of peer review. We portray peer review to the public as a quasi-sacred process that helps to make science our most objective truth teller. But we know that the system of peer review is biased, unjust, unaccountable, incomplete, easily fixed, often insulting, usually ignorant, occasionally foolish, and frequently wrong.”
Ακόμα και οι ίδιοι συντάκτες των σημαντικών (ιατρικών) περιοδικών (journals) αναγνωρίζουν ότι η εξέταση από ομότιμους (peer review) πιθανόν δεν είναι το καλύτερο σύστημα που επινοήθηκε ποτέ από την ανθρωπότητα. Παρακάτω είναι τι είπε ο Richard Horton, ο αρχισυντάκτης του Lancet, πάνω στο θέμα:
" Το λάθος φυσικά είναι ότι έχουμε θεωρήσει ότι η εξέταση από ομότιμους ήταν κάτι παραπάνω από μία ωμή μέθοδος στο να διαπιστώνουμε την αποδεκτικότητα - όχι την εγκυρότητα - ενός νέου ευρήματος. Οι συντάκτες όσο και οι επιστήμονες, επιμένουν ότι είναι ζωτικής σημασίας η εξέταση από ομότιμους. Παρουσιάζουμε την εξέταση από ομότιμους στο ευρύ κοινό ως μια "ιερή" μέθοδο που μας βοηθάει να καταστήσουμε την επιστήμη ως τον πιο αμερόληπτο αφηγητή της αλήθειας. Αλλά ξέρουμε ότι το σύστημα της εξέτασης από ομότιμους είναι μεροληπτικό, άδικο, ατελές, εύκολα φτιαχτό, μη υπόλογο, συχνά προσβλητικό, συνήθως αδαές, περιστασιακά ανόητο και πολύ συχνά λάθος."
A view supported from a slightly different angle by Dr Marcia Angell, who was the editor of the New England Journal of Medicine for two decades. This was, and remains, the single most powerful and influential medical journal in the world. At least it is, when it comes to citations and impact factor:
“It is simply no longer possible to believe much of the clinical research that is published, or to rely on the judgment of trusted physicians or authoritative medical guidelines. I take no pleasure in this conclusion, which I reached slowly and reluctantly over my two decades as an editor of The New England Journal of Medicine.”
As for the quality of published research itself, here is one of my favourite quotes by Drummond Rennie, at the time the Deputy Editor of the Journal of the American Medical Association:
“There seems to be no study too fragmented, no hypothesis too trivial, no literature citation too biased or too egotistical, no design too warped, no methodology too bungled, no presentation of results too inaccurate, too obscure, and too contradictory, no analysis too self serving, no argument too circular, no conclusions too trifling or too unjustified, and no grammar and syntax too offensive for a paper to end up in print.”[78]
from a talk by Richard Smith (ex-editor of the BMJ):[79]
“Examples of Methods for Pharmaceutical Companies to Get the Results They Want from Clinical Trials:
Conduct a trial of your drug against a treatment known to be inferior;
Trial your drugs against too low a dose of a competitor drug;
Conduct a trial of your drug against too high a dose of a competitor drug (making your drug seem less toxic);
Conduct trials that are too small to show differences from competitor drugs;
Use multiple endpoints in the trial and select for publication those that give favourable results;
Do multicentre trials and select for publication, results from centres that are favourable;
Conduct subgroup analyses and select for publication those that are favourable;
Present results that are most likely to impress – for example, reduction in relative rather than absolute risk.
“The British Medical Journal (BMJ) has alleged that pharmaceutical giant Roche is deliberately hiding clinical trial data about the efficacy of oseltamivir (Tamiflu) in patients with influenza. The journal says global stockpiling and routine use of the drug are not supported by solid evidence and alleges that Roche concealed neurological and psychiatric adverse events associated with the neuraminidase inhibitor drug.
“In an open letter from Fiona Godlee, MD, editor-in-chief of BMJ, to Professor John Bell, FRS, HonFREng, PMedSci, Regius Professor of Medicine at Oxford University in the United Kingdom and a Roche board member, published online December 2012, Dr Godlee reminds Bell of concerns that were initially voiced in 2009 about the reliability of Tamiflu research.
“At that time, BMJ published an updated Cochrane review of neuraminidase inhibitors in healthy adults. That study ‘took the view that, since eight of the 10 [randomized controlled trials] on which effectiveness claims were based, were never published, and because the only two that had been published were funded by Roche and authored by Roche employees and Roche-paid external experts, the evidence could not be relied upon,’ Dr Godlee writes.”
“Academics with competing interests were nearly six times as likely as those without industry links (p=0.009) to predict a higher risk to the public from the pandemic than was given by official agencies… Academics who promoted the use of antiviral drugs in the UK media during the 2009-10 HIN1 flu pandemic were eight times as likely to have links with the drug industry as quoted academics who didn’t comment on their use.”
http://jech.bmj.com/content/early/2013/ ... 03128.full
“‘Journals have devolved into information laundering operations for the pharmaceutical industry’, wrote Richard Horton, editor of The Lancet, in March 2004. In the same year, Marcia Angell, former editor of the New England Journal of Medicine, lambasted the industry for becoming ‘primarily a marketing machine’ and co-opting ‘every institution that might stand in its way.’
“Jerry Kassirer, another former editor of the New England Journal of Medicine, argues that the industry has deflected the moral compasses of many physicians, and the editors of PLoS Medicine have declared that they will not become ‘part of the cycle of dependency …between journals and the pharmaceutical industry.’
“Twenty years ago this week the statistician Doug Altman published an editorial in the BMJ arguing that much medical research was of poor quality and misleading. In his editorial entitled, ‘The Scandal of Poor Medical Research,’ Altman wrote that much research was ‘seriously flawed through the use of inappropriate designs, unrepresentative samples, small samples, incorrect methods of analysis, and faulty interpretation.’ Twenty years later I fear that things are not better but worse…
“…’The poor quality of much medical research is widely acknowledged,’ wrote Altman, ‘yet disturbingly the leaders of the medical profession seem only minimally concerned about the problem and make no apparent efforts to find a solution.’
“Altman’s conclusion was: ‘We need less research, better research, and research done for the right reasons. Abandoning using the number of publications as a measure of ability would be a start.’
“Sadly, the BMJ could publish this editorial almost unchanged again this week. Small changes might be that ethics committees are now better equipped to detect scientific weakness and more journals employ statisticians. These quality assurance methods don’t, however, seem to be working as much of what is published continues to be misleading and of low quality. Indeed, we now understand that the problem doesn’t arise from amateurs dabbling in research but rather from career researchers.”
Richard Smith finishes this particular blog…
“I reflect on all this in a very personal way. I wasn’t shocked when we published Altman’s editorial because I’d begun to understand about five years’ before that much research was poor. Like Altman I thought that that was mainly because too much medical research was conducted by amateurs. It took me a while to understand that the reasons were deeper. In January 1994 at age 41, when we published Altman’s editorial, I had confidence that things would improve. In 2002 I spent eight marvellous weeks in a 15th century palazzo in Venice writing a book on medical journals, the major outlets for medical research, and reached the dismal conclusion that things were badly wrong with journals and the research they published. I wondered after the book was published if I’d struck too sour a note, but now I think it could have been sourer. My confidence that ‘things can only get better’ has largely drained away, but I’m not a miserable old man. Rather I’ve come to enjoy observing and cataloguing human imperfections, which is why I read novels and history rather than medical journals.”
υ.γ σεντόνι βγήκε
Επεξ/σία 11 Μαϊου από Unholydiver
Δεν είπα ότι είναι γεμάτη ηλίθιους. Είναι πολλοί όμως και είναι πολύ χαμηλό το μορφωτικό επίπεδο τους. Μην επεκταθώ στα υπόλοιπα.
Δεν είπα ότι συμβαίνει μόνο στην Ελλάδα. Συμβαίνει παντού, αλλού περισσότερο αλλού λιγότερο.
Προφανώς δεν είπα ότι είμαι "απλοϊκός εξυπνάκιας". Δικός σου προσδιορισμός είναι, γιατί δεν σου άρεσε το κυνικό και όχι ελιτίστικο σχόλιο μου.
Είπα το απλό. Βλέποντας τι υπάρχει γύρω σου, ακόμα και σε forum, καταλαβαίνεις γιατί έχεις πετύχει, όπου έχεις πετύχει.
Το αν περνάω τον πήχη των ψεκασμένων 2 φορές ή 100 φορές δεν το ξέρεις. Σίγουρα όμως τον περνάω εύκολα.
Δεν λέω γιατρουδάκο τον Τσιόδρα και έχω την αντίληψη να ξεχωρίσω δημοσίευση στο "New England Journal of Medicine" από ψεκασμένα blog.
Όχι φίλε, σε όσους έχουν διαφορετική άποψη μας αποκαλείτε ηλίθιους και μας προσάπτετε διάφορα επίθετα όπως 5Gάκηδες, ανεγκέφαλους, αντιεμβολιαστές, αρνητές ιών ( έχετε μεγάλη φαντασία) φτωχαδάκια (έυγε) και ένα σωρό.
Αφού έχεις την αντίληψη να ξεχωρίσεις δημοσίευση στα περιβόητα ιατρικά journals σε σχέση με εμάς τους ψεκασμένους, πάρε κάτι ωραίες δηλώσεις από κάποιους ψεκασμένους συντάκτες των journals. Να με συγχωρήσετε όσοι δεν γνωρίζετε καλά αγγλικά γιατί δεν προλαβαίνω να μεταφράσω παρά μόνο την πρώτη δήλωση, είμαι κουρασμένος και αγχωμένος που θα ανοίξω αύριο το μαγαζί.
.Even the editors of main journals themselves recognise that peer review may not be the best system ever devised by mankind. Here is what Richard Horton, the editor of The Lancet, has to say on the matter:
“The mistake, of course, is to have thought that peer review was any more than a crude means of discovering the acceptability — not the validity — of a new finding. Editors and scientists alike insist on the pivotal importance of peer review. We portray peer review to the public as a quasi-sacred process that helps to make science our most objective truth teller. But we know that the system of peer review is biased, unjust, unaccountable, incomplete, easily fixed, often insulting, usually ignorant, occasionally foolish, and frequently wrong.”
Ακόμα και οι ίδιοι συντάκτες των σημαντικών (ιατρικών) περιοδικών (journals) αναγνωρίζουν ότι η εξέταση από ομότιμους (peer review) πιθανόν δεν είναι το καλύτερο σύστημα που επινοήθηκε ποτέ από την ανθρωπότητα. Παρακάτω είναι τι είπε ο Richard Horton, ο αρχισυντάκτης του Lancet, πάνω στο θέμα:
" Το λάθος φυσικά είναι ότι έχουμε θεωρήσει ότι η εξέταση από ομότιμους ήταν κάτι παραπάνω από μία ωμή μέθοδος στο να διαπιστώνουμε την αποδεκτικότητα - όχι την εγκυρότητα - ενός νέου ευρήματος. Οι συντάκτες όσο και οι επιστήμονες, επιμένουν ότι είναι ζωτικής σημασίας η εξέταση από ομότιμους. Παρουσιάζουμε την εξέταση από ομότιμους στο ευρύ κοινό ως μια "ιερή" μέθοδο που μας βοηθάει να καταστήσουμε την επιστήμη ως τον πιο αμερόληπτο αφηγητή της αλήθειας. Αλλά ξέρουμε ότι το σύστημα της εξέτασης από ομότιμους είναι μεροληπτικό, άδικο, ατελές, εύκολα φτιαχτό, μη υπόλογο, συχνά προσβλητικό, συνήθως αδαές, περιστασιακά ανόητο και πολύ συχνά λάθος."
A view supported from a slightly different angle by Dr Marcia Angell, who was the editor of the New England Journal of Medicine for two decades. This was, and remains, the single most powerful and influential medical journal in the world. At least it is, when it comes to citations and impact factor:
“It is simply no longer possible to believe much of the clinical research that is published, or to rely on the judgment of trusted physicians or authoritative medical guidelines. I take no pleasure in this conclusion, which I reached slowly and reluctantly over my two decades as an editor of The New England Journal of Medicine.”
As for the quality of published research itself, here is one of my favourite quotes by Drummond Rennie, at the time the Deputy Editor of the Journal of the American Medical Association:
“There seems to be no study too fragmented, no hypothesis too trivial, no literature citation too biased or too egotistical, no design too warped, no methodology too bungled, no presentation of results too inaccurate, too obscure, and too contradictory, no analysis too self serving, no argument too circular, no conclusions too trifling or too unjustified, and no grammar and syntax too offensive for a paper to end up in print.”[78]
from a talk by Richard Smith (ex-editor of the BMJ):[79]
“Examples of Methods for Pharmaceutical Companies to Get the Results They Want from Clinical Trials:
Conduct a trial of your drug against a treatment known to be inferior;
Trial your drugs against too low a dose of a competitor drug;
Conduct a trial of your drug against too high a dose of a competitor drug (making your drug seem less toxic);
Conduct trials that are too small to show differences from competitor drugs;
Use multiple endpoints in the trial and select for publication those that give favourable results;
Do multicentre trials and select for publication, results from centres that are favourable;
Conduct subgroup analyses and select for publication those that are favourable;
Present results that are most likely to impress – for example, reduction in relative rather than absolute risk.
“The British Medical Journal (BMJ) has alleged that pharmaceutical giant Roche is deliberately hiding clinical trial data about the efficacy of oseltamivir (Tamiflu) in patients with influenza. The journal says global stockpiling and routine use of the drug are not supported by solid evidence and alleges that Roche concealed neurological and psychiatric adverse events associated with the neuraminidase inhibitor drug.
“In an open letter from Fiona Godlee, MD, editor-in-chief of BMJ, to Professor John Bell, FRS, HonFREng, PMedSci, Regius Professor of Medicine at Oxford University in the United Kingdom and a Roche board member, published online December 2012, Dr Godlee reminds Bell of concerns that were initially voiced in 2009 about the reliability of Tamiflu research.
“At that time, BMJ published an updated Cochrane review of neuraminidase inhibitors in healthy adults. That study ‘took the view that, since eight of the 10 [randomized controlled trials] on which effectiveness claims were based, were never published, and because the only two that had been published were funded by Roche and authored by Roche employees and Roche-paid external experts, the evidence could not be relied upon,’ Dr Godlee writes.”
“Academics with competing interests were nearly six times as likely as those without industry links (p=0.009) to predict a higher risk to the public from the pandemic than was given by official agencies… Academics who promoted the use of antiviral drugs in the UK media during the 2009-10 HIN1 flu pandemic were eight times as likely to have links with the drug industry as quoted academics who didn’t comment on their use.”
http://jech.bmj.com/content/early/2013/ ... 03128.full
“‘Journals have devolved into information laundering operations for the pharmaceutical industry’, wrote Richard Horton, editor of The Lancet, in March 2004. In the same year, Marcia Angell, former editor of the New England Journal of Medicine, lambasted the industry for becoming ‘primarily a marketing machine’ and co-opting ‘every institution that might stand in its way.’
“Jerry Kassirer, another former editor of the New England Journal of Medicine, argues that the industry has deflected the moral compasses of many physicians, and the editors of PLoS Medicine have declared that they will not become ‘part of the cycle of dependency …between journals and the pharmaceutical industry.’
“Twenty years ago this week the statistician Doug Altman published an editorial in the BMJ arguing that much medical research was of poor quality and misleading. In his editorial entitled, ‘The Scandal of Poor Medical Research,’ Altman wrote that much research was ‘seriously flawed through the use of inappropriate designs, unrepresentative samples, small samples, incorrect methods of analysis, and faulty interpretation.’ Twenty years later I fear that things are not better but worse…
“…’The poor quality of much medical research is widely acknowledged,’ wrote Altman, ‘yet disturbingly the leaders of the medical profession seem only minimally concerned about the problem and make no apparent efforts to find a solution.’
“Altman’s conclusion was: ‘We need less research, better research, and research done for the right reasons. Abandoning using the number of publications as a measure of ability would be a start.’
“Sadly, the BMJ could publish this editorial almost unchanged again this week. Small changes might be that ethics committees are now better equipped to detect scientific weakness and more journals employ statisticians. These quality assurance methods don’t, however, seem to be working as much of what is published continues to be misleading and of low quality. Indeed, we now understand that the problem doesn’t arise from amateurs dabbling in research but rather from career researchers.”
Richard Smith finishes this particular blog…
“I reflect on all this in a very personal way. I wasn’t shocked when we published Altman’s editorial because I’d begun to understand about five years’ before that much research was poor. Like Altman I thought that that was mainly because too much medical research was conducted by amateurs. It took me a while to understand that the reasons were deeper. In January 1994 at age 41, when we published Altman’s editorial, I had confidence that things would improve. In 2002 I spent eight marvellous weeks in a 15th century palazzo in Venice writing a book on medical journals, the major outlets for medical research, and reached the dismal conclusion that things were badly wrong with journals and the research they published. I wondered after the book was published if I’d struck too sour a note, but now I think it could have been sourer. My confidence that ‘things can only get better’ has largely drained away, but I’m not a miserable old man. Rather I’ve come to enjoy observing and cataloguing human imperfections, which is why I read novels and history rather than medical journals.”
υ.γ σεντόνι βγήκε
Επεξ/σία 11 Μαϊου από Unholydiver
Η αστρολογία είναι περισσότερο έγκυρη από τη διατροφική "επιστήμη" 
-
- Παραπλήσια Θέματα
- Απαντήσεις
- Προβολές
- Τελευταία δημοσίευση
-
- 77 Απαντήσεις
- 1912 Προβολές
-
Τελευταία δημοσίευση από Ιδεολόγος
13 Ιούλ 2024, 21:04
-
-
Νέα δημοσίευση Η αρχή του τέλους για τους τούρκους στην Κύπρο!!
από Unique » 13 Μαρ 2025, 07:21 » σε Εθνικά Θέματα - 36 Απαντήσεις
- 743 Προβολές
-
Τελευταία δημοσίευση από Αλιόσα
13 Μαρ 2025, 18:33
-
-
- 4 Απαντήσεις
- 225 Προβολές
-
Τελευταία δημοσίευση από JiGeZa
30 Ιαν 2024, 18:42
-
-
Νέα δημοσίευση Επαναπατρισμενοι Φορουμιτες, πως ειναι η εμπειρια της επιστροφης;
από Portia » 12 Ιούλ 2024, 13:10 » σε Κοινωνικά θέματα - 24 Απαντήσεις
- 737 Προβολές
-
Τελευταία δημοσίευση από Stinger
16 Ιούλ 2024, 17:29
-
